Summer 2012: Black foot disease
Regional Report Ventura and Santa Barbara Counties by Julie Newman
Black foot disease has caused significant economic damage in California grape vineyards since the 1990s. Young vines are most susceptible. Plant symptoms are black, sunken root lesions, necrotic root crowns and black vascular streaking. Leaves appear scorched or water stressed and may be chlorotic. Vines are often stunted and may be killed.
Black foot disease in grapes in California is caused by various species of Cylindrocarpon. The primary source of inoculum is infected soil, but nursery plants are also a potential infection source. In a 2007 survey of 165 non-symptomatic rooted grapevine cuttings selected randomly from various nurseries in California throughout the year, 26% tested positive for Cylindrocarpon in PCR assays.
Cylindrocarpon is a common soil-borne fungus that causes root rots and rots of underground storage structures such as bulbs. These fungi are often associated with other pathogens and have a wide host range, including various agricultural crops such as fruit trees (e.g., apple, peach, plum) and strawberries (fig. 1), in addition to grapes. Many ornamental species are Cylindrocarpon hosts, including boxwood (fig. 2) — not to be confused with Cylindrocladium that causes boxwood blight — and other woody shrubs such as azalea (fig. 3). Various trees (e.g., pine, douglas fir, deodar cedar, magnolia) and flowering plants (e.g., African violet, cyclamen, Easter lily, oriental lily) are hosts. In California, there are three common Cylindrocarpon species: C. destructans, C. liriodendri and C. macrodidymum. Of 192 California Department of Food and Agriculture state records of Cylindrocarpon found on 124 host species since 2007, over 74% were found in Santa Barbara County and 10% were found on nursery stock.
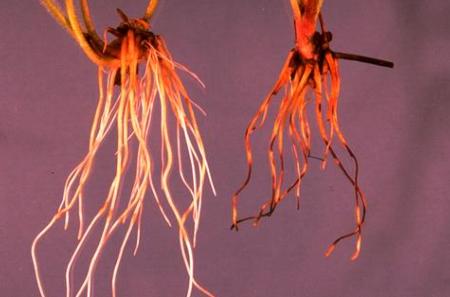
Fig. 1. Black root rot damage on the strawberry plant on the right caused by Pythium and Cylindrocarpon spp. Photo by Paul Reeser.

Fig. 2. These Japanese boxwood (Buxus microphylla japonica) nursery plants are infected with Cylindrocarpon sp. Symptoms shown include poor growth, low vigor, yellow foliage and defoliation. Photo by Heather Scheck, Santa Barbara County Agricultural Commissioner.

Fig. 3. Healthy azalea roots (left) compared to roots infected with Cylindrocarpon destructans. Damage symptoms shown include low root volume and dark discoloration. Not shown are the above-ground symptoms which include stunted and weak growth. Photo courtesy of the California Department of Food and Agriculture.
Cylindrocarpon is spread in the nursery in contaminated potting media or soil. Potting media should be heat pasteurized or solarized; methyl bromide is an effective soil treatment but is being phased out. Equipment and reused containers can also spread the disease and should be thoroughly washed to remove clinging debris, soil or potting mix particles, and plant material. Heat treatment is effective in killing the plant pathogens that adhere to containers or that are in the debris. Where steam is not available, solarization may be effective. Hot water dips have been shown to be effective in reducing Cylindrocarpon inoculum on containers and in infected nursery stock. DMI fungicides (e.g., triadimefon, propiconazole, myclobutanil) and thiophanate-methyl are effective fungicides for managing disease problems (be sure to check labels for registration and use requirements). Biocontrol studies using mycorrhizal fungi resulted in good control of black foot disease in grapes.
References
British Columbia Ministry of Agriculture 2010. Black foot. In: Best Practices Guide for Grapes. British Columbia Ministry of Agriculture and the British Columbia Wine Grape Council. Vancouver, B.C. http://www.agf.gov.bc.ca/cropprot/grapeipm/cylindrocarpon.htm.
Dubrovsky S, Fabritius A. 2007. Occurrence of Cylindrocarpon spp. in nursery grapevines in California. Phytopathologia Mediterranea 46(1): 84–86.
Dumroese R K, James RL, Wenny DL. 2002. Hot water and copper coatings in reused containers decrease inoculum of Fusarium and Cylindrocarpon and increase Douglas fir seedling growth. HortScience 37(6): 943-947. http://hortsci.ashspublications.org/content/37/6/943.full.pdf.
Gubler D. 2004. Identification and Control of Cylindrocarpon Black Foot Disease in California. Research Summary AVF Proj. ID: 276. American Vineyard Foundation. Napa, CA http://www.avf.org/article.html?id=3381.
Koike ST, Wilen CA. 2010. Management of soilborne pathogens. In: UC IPM Pest Management Guidelines: Floriculture and Ornamental Nurseries. UC ANR Publication 3392. University of California, Davis, CA. http://www.ipm.ucdavis.edu/PMG/r280190211.html.
Scheck H. 2012. Cylindrocarpon Black Foot Disease. PowerPoint Presentation. Santa Barbara County Agricultural Commissioner’s Office. Santa Barbara, CA.
Scheck H, Vasquez S, Fogle D, Gubler W D. 1998. Grape growers report losses to black-foot and grapevine decline. California Agriculture 52(4):19-23.
Julie P Newman
Floriculture and Nursery Crops Advisor
UC Cooperative Extension Ventura County
669 County Square Drive, #100
Ventura, CA 93003-5401
(805) 645-1459 phone
(805) 645-1474 fax
jpnewman@ucdavis.edu
http://ceventura.ucdavis.edu












