UC demonstrates benefits of a scouting program for insects and mites
by James Bethke
During my first few years as an advisor with Cooperative Extension, I was contacted by a grower who was desperate because he could no longer kill one of his major pests, the twospotted spider mite. Obviously, the first thing I thought of was that there was miticide resistance. I knew in order to assist this grower, I was going to have to investigate his spray program and recommend a good rotational program. On my first visit, the problem was immediately apparent. On the wall outside his office was a routine spray program for a tank mix that included very few miticides, Orthene and fungicides. This tank mix was sprayed on all of his plants every Saturday, and the program was written out for an entire year.
Hopefully, you can see the issue right away. This kind of pest management program is fraught with problems and doomed to fail. The entire arsenal in his pesticide shed was exceptionally small. It was clear that he needed to increase the number of rotational products immediately. However, that was the least of his problems. Why spray all plants every time? Is every plant in the facility infested? It was clear that the grower needed some assistance in organizing an effective integrated pest management program. The most important components of IPM programs are scouting and basing pest management decisions on monitoring, rather than spraying on a calendar schedule. Therefore, our research group dedicated the time necessary to train the grower and his staff on how best to scout and treat major ornamental pests. This article describes the process and some of the results.
Mapping
The very first thing we did was to number all of the benches in the facility and create a map of the three growing areas. Fig. 1 represents of one of these growing areas. Using the maps and bench numbers, we could document plant types, plant movement and pest management trends. Following our mapping, we noticed that mite-sensitive plants such as dieffenbachia and neanthe bella palm were spread throughout all three growing areas. If the mite-sensitive plants were heavily infested, they were acting as a reservoir to infest less sensitive plants, thereby forcing the treatment of plants that were typically not infested with mites. We recommended grouping these mite-sensitive plants together. In this way, scouting and management practices could be intensified in these areas.
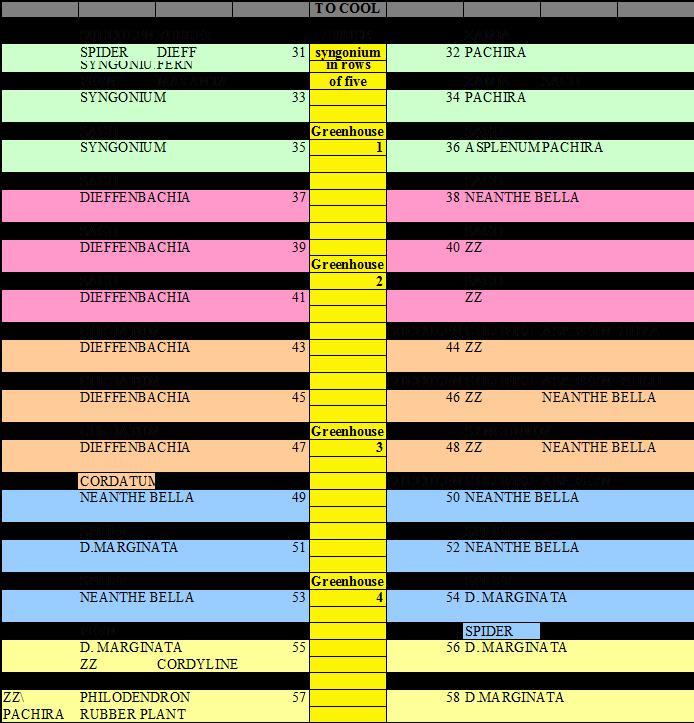
Fig. 1. Diagram of one of three growing areas at a cooperating grower’s facility. The growing area is all under one greenhouse roof with four peaks. The top of the diagram represents the cooling pad of a fan-and-pad system. The references to greenhouses 1–4 are the four peaks (four different colors) or houses within the growing area. The yellow area is the center aisle. The benches under each house are numbered 31 through 58. The plant types on each bench (within colored area) are listed with the bench number. The white area above each colored bench area references hanging plants above the respective bench.
Scout Team
Scout. We dedicated one of our UC staff as the scout who would use indirect sampling techniques (trapping the surrounding area using sticky cards, pheromone traps, black light traps, etc.) and direct sampling (sampling the actual plant, such as leaf and stem samples, visual observations of plant parts, tapping flowers on white paper, etc.) to monitor pest populations and locations. Further, one employee at the nursery facility would be trained and shadow the scout. It is very important to have trained, knowledgeable staff as pest management scouts. They should become familiar with specific problems on specific plants, seasonal trends and thresholds. We also brought a stereomicroscope from our lab to help us identify pests, and we eventually convinced the grower to purchase one because it is very useful in identifying pests and observing mortality following spray applications.
Workers. We asked the supervisors to train their staff to notify us of any unusual looking plants or plant parts that might be indicative of a pest problem. These employees know the plants well, and would be the most apt to see problems, as they have the most contact with the plants.
Decision Maker. I served as the decision maker, the person who recognized that the level of pest required control measures and decided what control measures were to be employed. I used the weekly pest report (see below) as a tool for decision making.
Sampling Methods
Indirect Sampling. Yellow sticky cards were not employed at the facility, so we hung a yellow card under each peak and on each side of the center aisle. These cards would give us an idea of the flying insect pests in the area and those that could impact plants at the facility. Caterpillars were never a problem for this grower, so we did not employ any pheromones or black lights at the facility. We found an area of fungus gnats that needed to be monitored weekly.
Direct Sampling. Indirect sampling does not tell the whole story. Finding numerous flying pests (adults) on a sticky card does not mean that there is a growing pest population (adults and immatures) on the plants. It is a relative measure and a seasonal trend indicator, an early warning system at times. Therefore, we also employed direct sampling techniques. The scout walked the entire facility in a day looking for any obvious pest infestations like aphids, randomly turning leaves over looking for pests common on the undersides such as whiteflies and mites. Leaves or plants with obvious pest damage were observed under the microscope in search of live or dead pests. Direct sampling helps identify specific pest locations, the relative level of infestation, and the most prevalent stage of development of the pest. The development stage may be important because each stage may require that a different pest control measure be employed. Further, direct sampling helps evaluate pest control measures.
Record Keeping
Keeping records will greatly increase your ability to identify pest trends that can help to make pest management decisions. We created a scouting form specific to this facility (fig. 2).
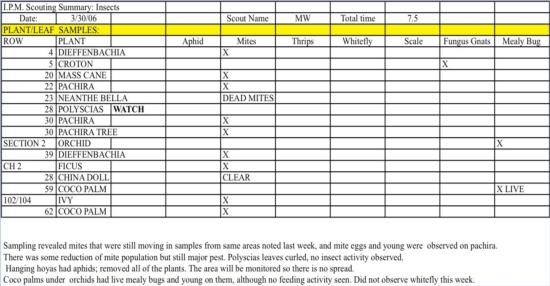
The form contained only information from infested areas and not from every area in every greenhouse, and the reports were typically short. The form contained several categories based on our mapping. There were three SECTIONs or greenhouses with multiple peaks or houses within (fig. 1 is an example of one section with four houses), and each ROW represents a numbered bench in a specific house. If we found a pest on a specific bench, we could find it on the map easily. If plants on a bench were infested, the plant type was noted under the category PLANT. The name of the scout and how much time was spent (hours) scouting is displayed above. Common insect and mite pests found in this type of production are listed as headers. An X represents the presence of the pest on that bench and on that plant, and sometimes descriptions are noted in the cells as to the viability of the pest or severity of the infestation. Finally, a written summary of what was found during the scouting, such as the severity of the infestation and other observations, is written below the scouting form.
Recommendations
Once I received the report from the scout, I was able to provide pest management options and, very importantly, recommend treatment of specific infestations, and not the whole facility. Fig. 3 is an example of one of those recommendations. Note that it includes a suggestion to increase the number of different types of miticides so that the grower could do a more effective job of resistance management.

Conclusions
By implementing scouting practices and focusing pesticide applications to the areas of need, we were able to demonstrate to the grower that he could reduce overall pesticide use. I felt, however, that it was critical that the grower increase the number of different chemical mode of actions because the mites in the facility were very tolerant to miticides. That initial increase in the number of pesticides increased costs initially, but the grower would benefit in the long run from better efficacy through good rotation of chemicals. The consistent spraying of a tank mix of a small number of products on a scheduled basis was causing the most serious problem at his facility. He is now employing a regimented rotation of miticides, and his mite resistance problem is under control. A good description of the miticides and rotation can be found at the following link on the UCNFA website: http://ucanr.edu/sites/UCNFA/files/181232.pdf.
As I mentioned before, the grower purchased a microscope and he and his staff use it regularly. Additionally, I was very encouraged to receive an email from him that indicated that he had improved upon our recording-keeping methods and mapping by creating his own personalized maps and forms. He got it!
James A. Bethke is County Director and Farm Advisor for Nurseries and Floriculture, UC Cooperative Extension, San Diego and Riverside Counties.












