Solarization: A Simple and Low-cost Method for Disinfesting Horticultural Containers
by Karen Suslow and Kathy Kosta
The reuse of dirty plant pots by nursery growers has repeatedly been shown to be a method by which plant pathogens are transferred within or to a nursery. More critically, this practice is an efficient pathway to infest landscape settings or habitat restoration sites by the out-planting of pre-symptomatic infected plant material. The transfer of water molds (Oomycetes), such as plant pathogenic Phytophthora species, is a major threat to nursery operations and prevention of cross-contamination via pots should be a critical nursery management goal. Our research has established performance and efficacy criteria that demonstrate the risk can easily be managed by the solarization of used pots.
Materials and Methods
In the summer of 2015, the National Ornamental Research Site at Dominican University of California (NORS-DUC) conducted outdoor solarization experiments designed to determine the temperature and time requirements at which Phytophthora cactorum, a commonly found soilborne plant pathogen in the nursery industry, would be killed. P. cactorum served in our trials as a surrogate, a test organism to determine the efficacy of sanitation regimes for P. ramorum (which causes sudden oak death)and P. tentaculata, plant pathogens which were shown to be killed at similar disinfection temperatures as P. cactorum.

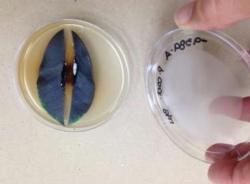
We used 1-gallon (1G), D-40 and Tubex Tubes, containers that are commonly used by small-scale and large-scale growers. Tubex Tubes are used to protect outplanted stock in restoration sites from browsing wildlife. In this trial, “treatments” are those containers wrapped tightly in clear polymer (plastic), bought off the shelf at a mass merchant store (fig. 1). “Controls” are the containers not wrapped in plastic. Trials were set up in two outdoor locations in California: in a hot climate (Winters, fig. 2) and in a cool climate on the coast (Pacifica).
Fig. 2. Field layout of treated (pots wrapped in clear plastic) and controls (pots not wrapped in clear plastic) at the Winters site. Photo: K. Suslow.
1G pots were nested 3 feet high and three stacks were secured together flat on the ground with gardening tape and laid on black plastic. Spectrum WatchDog Data loggers were inserted in the center stack, on the bottom-side of the centrally located pot, where we have demonstrated the coolest temperatures occur in the stack. When replicating this experiment at your facility, always ensure the data logger or temperature probe is in the coolest location so that the entire stack reaches the required temperatures. All pots in the treatments were sprayed with water prior to sealing them in plastic.
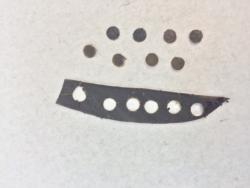
Leaf disks from P. cactorum-infected rhododendron leaves (fig. 3) were mixed with soil and placed inside narrow mesh sachets; three sachets were then inserted into a hollow-core rope (fig. 4), spaced 8 inches apart for easy weekly retrieval. The experiment was conducted over a three-week period.

Each week, one sachet was retrieved by extracting the rope through a hole drilled in the side of the nested 1G pots (fig. 5).
Fig. 5. A hole drilled in the side of the nested 1G pots enabled weekly extraction of sachet. Photo: K. Suslow.
Results and Conclusions
For all pot sizes and in all trials, pots laid horizontal onto black plastic on the ground yielded higher daytime and nighttime temperatures than the ambient temperatures. Although reaching the required temperatures for solarizing is more easily attained in the summer months, in cooler climates and in the later fall months, the radiant heat from the ground and the black plastic will aid in hastening the solarizing process. The treatments (those pots wrapped in clear plastic) can obtain a heat-capture gain of up to 200F (120C) as compared to the controls (pots not enclosed in a plastic).
Table 1. Cumulative hours attained during the first week of the Winters and Pacifica trials for 1G pots. Data after the first week is not shown because the pathogen was killed in the treated pots during the first week in both research sites.
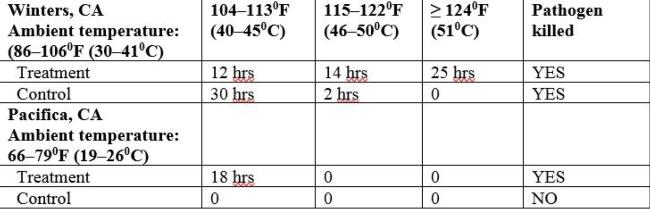
In lab studies, P. cactorum, as well as numerous other Phytophthora and Pythium species, can be killed at 1220F (500C) for 30 minutes when exposed to moist heat (Baker, KF and Cook RJ; Griesbach et al.). Reducing the temperature and extending the time has proven to be just as effective at killing P. ramorum. In infected rhododendron tissue, in loam soil, P. ramorum was not recovered after two days at 1040F (400C) nor at 4 days at 950F (350C) (Tooley et al). As noted in table 1, P. cactorum was killed in the treatments in the cool climate trial in Pacifica when the temperatures reached 104–1130F (40–450C) for 18 hours cumulatively over the first week of the experiment. Ambient temperatures did not exceed 790F (260C).
When solarizing used 1G pots in the warm months of the year, wet the stacked pots, seal them in plastic and align them in a single layer on the ground on a black tarp so that they are exposed fully to the sun. With a temperature probe in the coolest part of the stacked pots, confirm temperatures have reached 1220F (500C) for minimally 30 minutes; this can potentially be achieved within a day in a warm climate. Without a temperature probe, track the ambient temperature and when it reaches 1060F (410C) on a daily basis for a week, from the data in table 1 you can expect that your sealed pots have attained the necessary temperature to kill Phytophthora and Pythium species.
Future pot solarization work at NORS-DUC includes using the quarantine pathogens P. ramorum and P. tentaculata, trialing pot solarization on a large scale with a commercial nursery and configuring the pots in different layouts to increase the throughput.
Karen Suslow is Program Manager, National Ornamental Research Site at Dominican University of California (Karen.Suslow@dominican.edu); Kathy Kosta is Plant Pathologist, California Department of Food and Agriculture (Kathy.Kosta@cdfa.ca.gov).
References
Baker KF and Cook RJ. 1974. Biological Control of Plant Pathogens., SF: W.H. Freeman and Co.
Griesbach JA et al. 2012. Safe Procurement and Production Manual. A systems approach for the production of healthy nursery stock. ISBN 978-0-615-50862-7.
Tooley P, Browning M and Berner D. 2008. Recovery of Phytophthora ramorum following exposure to temperature extremes. Plant Dis. 92:431-437.












