Slow Sand Filters Remove Tobacco Mosaic Virus
by Loren Oki, Lloyd Nackley and Bruno Pitton, Sohrab Bodaghi, Eric Lee, Darren Haver and Deborah Mathews
Slow sand filters (SSF) are an old technology that utilizes a microbial community to degrade contaminants in water, including plant pathogens. The uncleaned water passes through a bed of sand that serves as a substrate for the microbial community, allowing a biofilm to form around the sand grains. As the filter’s microbial community matures, the surface of the sand bed becomes covered with a thick layer of microbes commonly referred to as the “schmutzdecke,” German for “dirt layer.” The exact treatment mechanisms are unclear, but plant pathogen removal is linked to the schmutzdecke. However, some removal of larger plant pathogens and other contaminants may be due to either physical filtering or adhesion to the sand itself. The properties of the sand bed and filtration system are important the to optimize filtration process:
- The sand should be uniform round grains about 0.3 to 0.6 mm in diameter.
- The sand bed should be approximately 3 feet deep to allow for removal of the top few centimeters of sand once the biofilm has thickened and flow is restricted.
- There should be about 3 feet of water above the sand bed to provide pressure, as gravity is the means for water flow.
- Controlling the flow rate through the sand bed is important to optimize treatment. If the flow rate is too high, treatment is ineffective.
- Pre-filtration of particulates prior to treatment will help prevent sand bed clogging and extend intervals between maintenance.
As the name implies, flow rates through the sand bed are slow. One square foot of sand bed area can treat 0.06 to 0.2 gallons per minute. So, a 12-foot diameter round tank containing a sand bed would be able to treat about 10,000 gallons per day at the lower flow rate. The containment used to hold the sand bed is not important and could be a water tank, septic tank, or lined reservoir, for example. A manifold is placed at the bottom of the containment to collect the treated water and is covered with pea gravel. Above the pea gravel is a series of layers of graduating sand size, from coarse to fine, to prevent the fine sand from entering the pea gravel. Above these layers is the filtration sand bed material. A pump is used to extract the treated water from the sand bed system into a storage tank.
Our previous work (Lee and Oki 2013) showed that SSFs are effective at removing Phytophthora when captured water was inoculated with the pathogen (fig. 1). However, Deborah Mathews, a plant pathologist formerly at UC Riverside, wanted to know if these filters could remove plant pathogenic viruses. The literature was unclear if SSFs could remove plant viruses (Krczal et al. 1995; van Os et al. 1998), although extensive work shows removal of viruses that affect bacteria and humans (Paranychianakisa et al. 2006). A pilot study was conducted at the UC ANR South Coast Research and Extension Center in Irvine, CA using a single sand filter made of 4-inch PVC pipe to see if Tobacco mosaic virus (TMV) removal was effective. Anaheim pepper plants were irrigated on benches in a greenhouse and the runoff was captured and pumped to the top of the filter system.

Before inoculation of TMV, water samples from the filter were retrieved from above the sand bed (pretreatment) and after the water had passed through the bed (post treatment) to get background measurements (Day 0). After the water samples were collected, a suspension of TMV was added to the water at the top of the filter three days per week. Water samples were collected 24 hours after the initial TMV addition and at 7-day intervals thereafter for a total duration of 12 weeks. The water samples were analyzed using ELISA and bioassays in which Nicotiana glutinosa, Chenopodium quinoa, N. benthamiana and N. tabacum were inoculated with pre- and post-treatment water and evaluated for symptoms. N. glutinous and C. quinoa were local lesion/hypersensitive response bioassays, whereas N. benthamiana and N. tabacum were systemic whole plant assays.
Both the ELISA and bioassays showed that the TMV was removed after about 9 weeks. The pilot study was repeated using three filters, but only the whole plant assays were used along with the ELISA analyses. Again, TMV was removed from the filtered water after about 6 weeks (table 1.). The faster removal could be attributed to warmer temperatures as the second study occurred later in the season compared to the first.
Plant viruses, including TMV (Hong and Moorman 2005), have been recovered from irrigation runoff, so their spread through the reuse of this water is highly likely. TMV is known to be a very robust virus that can survive for very long periods in a wide variety of conditions and its removal means that the removal of other plant pathogenic viruses is likely.
Also see: Oki, L. 2013. Update of slow sand filtration research. UCNFA News. 17(2): 4-6. http://ucnfanews.ucanr.edu/Articles/Feature_Stories/Update_of_Slow_Sand_Filtration_Research/.
Loren Oki is UC Cooperative Extension Landscape Horticulture Specialist, Department of Plant Sciences, UC Davis; Lloyd Nackley is Post-Doctoral Scholar, Department of Plant Sciences, UC Davis; Bruno Pinon is Staff Research Associate, Department of Plant Sciences, UC Davis; Sohrab Bodaghi is Associate Research Scientist, Department of Plant Pathology, UC Riverside; Eric Lee is Science Research Analyst, SusCon; Darren Haver is Director UC Cooperative Extension, Orange County; and Deborah Mathews is CE Specialist (Retired), Department of Plant Pathology, UC Riverside.
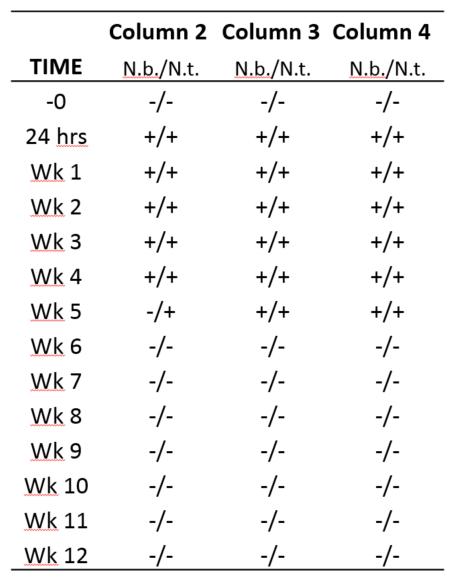
collected before TMV was added to the SSFs. Plants used for the assays were Nicotiana benthamiana (N.b.) and N. tabacum (N.t.). At Week 6 and therafter, all of the assays were negative indicating no presence of TMV.
References
Hong CX, Moorman GW. 2005. Plant pathogens in irrigation water: Challenges and opportunities. Critical Reviews in Plant Sciences 24: 189-208.
Krczal G, Albouy J, Damy I, Kusiak C, Deogratias JM, Moreau JP, Berkelmann B, Wohanka W. 1995. Transmission of pelargonium flower break virus (PFBV) in irrigation systems and by thrips. Plant Disease 79: 163-166.
Lee E, Oki LR. 2013. Slow sand filters effectively reduce Phytophthora after a pathogen switch from Fusarium and a simulated pump failure. Water Research 47: 5121-5129. doi: 10.1016/j.watres.2013.05.054.
Paranychianakisa NV, Angelakisa AN, Leverenz H, Tchobanoglous G. 2006. Treatment of wastewater with slow rate systems: A review of treatment processes and plant functions. Critical












