Nutrient Release from Controlled-Release Fertilizers in Nursery Production Systems
by Donald J. Merhaut, Eugene K. Blythe, Joseph P. Albano, and Julie P. Newman
Federal guidelines have been in place since 1972 to mitigate pollutants entering surface water. In addition, the state of California has developed regulations associated with pollutants entering the watersheds. Regulations are based on the pollutants impairing each waterbody or watershed. Of the agricultural pollutants being regulated, nitrate and phosphate are the main fertilizers of concern. Nitrate is the most likely to appear in runoff since it is applied in the greatest quantities relative to other nutrients. Also, nitrate is a negatively charged compound (anion) and, therefore, does not readily bind to the predominantly negatively charged surfaces of soil and substrate particles. Similarly, because phosphate is also an anion, it may leach readily from soil or growing media. Many state water agencies have now implemented regulations to mitigate nitrate and phosphate runoff from agricultural sites and urban greenbelts. Other essential plant nutrients, such as iron, manganese, copper and zinc, are also listed in the Clean Water Act. However, in most regions, these nutrients are not found in quantities that are considered as pollutants for water bodies; therefore, regulations regarding these nutrients have not been needed.
Over the past 70 years, coated fertilizers have been developed that allow a slow release of nutrients into the planting medium throughout the period of crop growth. Most of these controlled-release fertilizers (CRFs) are coated with polymers which release nutrients at a rate that is positively correlated with increasing media temperatures, within a certain range of temperatures. Short-term (less than 4-month) studies have previously shown nutrient release characteristics under controlled environmental conditions. In the following two studies, we monitored nutrient release characteristics from CRFs in a greenhouse environment and an outdoor environment during an 11-month production period, a time frame that is typical for production of many woody ornamental plants. Based on these studies, which we summarize below, there are several best management practices (BMPs) that can be adopted when using CRFs so that growers can optimize nutrient uptake into crops while simultaneously minimizing the likelihood of nutrient leaching from the containers. For more details from these studies, please refer to the articles published by the American Society for Horticultural Science listed in the references.
Experiments
Studies were conducted in Riverside, California, a region experiencing rapid growth in wholesale nursery production. The desert climate has summer and winter high temperatures, averaging in the high 90s (°F) and upper 60s to low 70s, respectively. Rainfall occurs during the winter months. The studies were initiated in August and ended in June of the following year.
We tested four fertilizer brands: (1) Multicote 17-5-11 plus minors, (2) Nutricote 18-6-8 Total, (3) Osmocote 24-4-9 plus the addition of Micromax, and (4) Polyon 17-5-11 plus micros. All fertilizers were one-year release formulations; however, release rates of these CRFs are based on different temperature regimes: 80°F for Osmocote and Polyon, 70°F for Multicote, and 70 to 80°F for Nutricote. Elemental concentrations and compounds used in each fertilizer were different (table 1). Since the percentage of nutrients contained in the four fertilizers varied, we normalized fertilizer applications so that all treatments received the same amount of nitrogen.
Table 1. Amount (% by weight) of nitrogen (N), phosphorus (P) and potassium (K) in Multicote 17-5-11 + minors, Nutricote 18-6-8 total, Osmocote 24-4-9, and Polyon 17-5-11 + micros.
|
Fertilizer |
Ammonium-N |
Nitrate-N |
Urea-N |
Total N |
Phosphorus |
Potassium |
|
Multicote |
9.00 |
8.00 |
0.00 |
17.00 |
2.10 |
9.10 |
|
Nutricote |
8.60 |
9.40 |
0.00 |
18.00 |
2.52 |
6.62 |
|
Osmocote |
6.40 |
5.70 |
11.90 |
24.00 |
1.68 |
7.44 |
|
Polyon |
7.30 |
9.70 |
0.00 |
17.00 |
2.02 |
8.85 |
Both experiments were conducted using growing media in 1-gallon black polyethylene pots. A “no plants” treatment was used in these experiments as a control so that all leachate that drained and was collected from the bottom of the pots would contain essentially all nutrients released from the CRFs as well as some nutrients released from the growing medium as the medium broke down. Containers were irrigated by drip irrigation and leachate was collected immediately after each irrigation. Nutrient concentrations, electrical conductivity (EC) and pH of leachate were measured on a weekly basis.
Greenhouse Experiment
The indoor study was conducted in a ventilated, unheated greenhouse representing conditions typically used to grow ferns, azaleas and camellias in southern California. An acid growing medium consisting of peat, pine bark and sand was prepared with CRF fertilizer blended into the mix. Fertilizer was added at a low rate, 3.3 g nitrogen per 1-gallon pot, which is an average rate based on recommendations by the four fertilizer manufacturers for these crops, all of which have low nutrient requirements and low tolerance of high salt levels. Air temperatures ranged between 75 to 86°F from the beginning of August to mid-October and again from mid-April to the end of June. From mid-October until mid-April greenhouse temperatures averaged below 70°F (fig. 1).
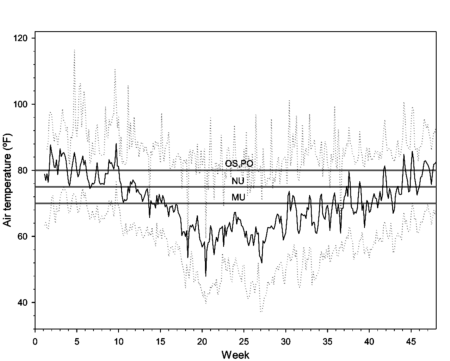
Fig. 1. Daily average (solid line), minimum (lower dotted line) and maximum (upper dotted line) greenhouse air temperatures over a 47-week period (1 Aug. to 27 June), with gray horizontal lines indicating media temperatures for 12-month fertilizer release as specified by the respective manufacturers of Osmocote (OS), Polyon (PO), Multicote (MU), and Nutricote (NU).
Outdoor Experiment
The outdoor study was conducted on raised benches in full sun and ambient temperatures. A neutral-pH medium consisting of composted forest products, sand and pine bark was prepared and fertilizer was added a higher rate, 6.6 g nitrogen per 1-gallon pot, an average rate based on recommendations by the four fertilizer manufacturers for plants that have higher nutrient demands. Outdoor air temperatures (data not shown) fluctuated in a manner similar to the greenhouse study, but outdoor summer highs and winter lows were more extreme.
Results
Only data from the greenhouse study is presented, since trends were somewhat similar with the outdoor experiment. During the first 10 weeks of both studies, the EC was relatively high (fig. 2). EC was somewhat higher in the outdoor experiment since fertilizer rates were twice that of the greenhouse study. EC of leachates is associated with both the leaching of salts already present in the media and the release and leaching of nutrients from the fertilizer prills. The high EC during the first two months of the studies occurred during the period when air temperatures were relatively high, so one would expect a greater release of nutrients from the fertilizer prills since release characteristics are temperature dependent. Leachate concentrations of total inorganic nitrogen (ammonium and nitrate) (fig. 3) and total phosphorus (fig. 4) were relatively high and fluctuated somewhat during the first 20 weeks of the study. Nutrient concentrations of leachates then decreased and remained relatively stable throughout the last 27 weeks of both studies.
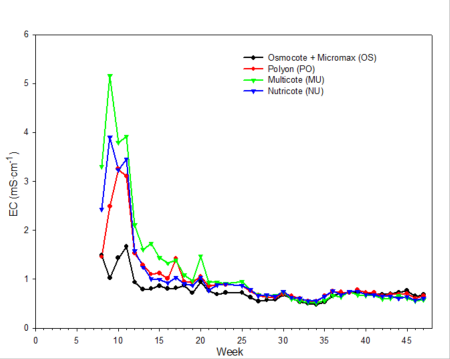
Fig. 2. Electrical conductivity (EC) of irrigation leachates collected weekly during the study period from pots containing one of four different CRFs.
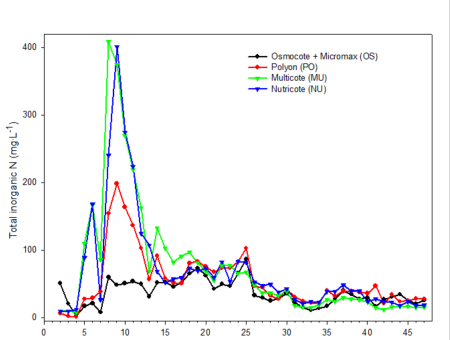
Fig. 3. Inorganic nitrogen (nitrate + ammonium) concentrations in irrigation leachates collected from one gallon containers weekly during the experiment.
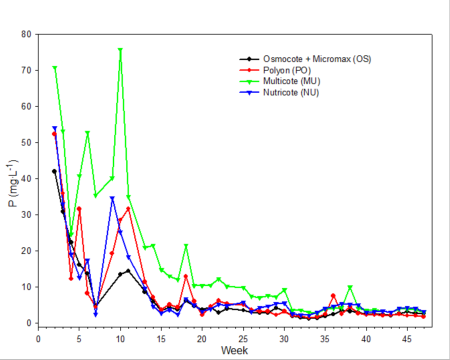
Fig. 4. Total phosphorus concentrations in irrigation leachates collected from one gallon containers weekly during the experiment.
Conclusions
There are several important points to be gathered from these studies:
Nutrient release from CRFs increases with increasing temperature.Nutrient release from CRFs in container growing media can be relatively high (at least for the products tested) if planting is done during hot summer months.
Electrical conductivity (EC) of leachate is not directly correlated with nutrient content.Nonessential salts such as sodium, as well as other breakdown products of the growing medium, will contribute to the EC of the leachate. In addition, some fertilizers, such as urea, have a low salt index; therefore, assumptions cannot be made that EC is directly associated with the concentration of any one particular element (compare fig. 2 [EC] with fig. 3 [inorganic nitrogen] and fig. 4 [phosphorus]). However, with experience and use of long-term records of fertilizer types used, type of growing media and environmental conditions, EC can be used as a general guideline for estimating nutrient levels.
Measurements of chemical characteristics of leachates from pots of growing media will vary from week to week and from container to container.Even in controlled studies, such as the studies described here in which no plants were used, EC and nutrient concentrations of leachates fluctuated within a given treatment, especially during the first half of the study period. Therefore, if collecting samples and making cultural management decisions based upon leachate data, it is important to collect leachate samples from several containers (rather than a single container) for testing. Sampling and testing should also be done on a regular basis and a record of the results should be maintained so that changes over time can be used to make cultural management decisions.
Best Management Practices
Based on these conclusions, several BMPs should be considered when using CRFs.
BMP1: Canning.Prepare growing media and plant crops in the cooler periods of the year when possible. This will allow roots to develop throughout the container. As temperatures warm, nutrients released from CRFs will be taken up by the already present root system.
BMP2: Irrigation and weather. Minimize water runoff from containers by avoiding excessive irrigation volumes during each irrigation event. This is especially important during warm or hot weather, when the rate of nutrient release from CRF prills is higher.
BMP4: Irrigation and new plantings.Newly planted crops (plugs or liners transplanted into larger containers) have special water and fertilizer needs. Until root systems become established in containers, any water and nutrients in excess of that needed by the plants will end up as runoff.
BMP5: Media storage.If CRFs are blended into the growing media, prepare only enough media for immediate use. The CRF prills in any unused media will begin releasing nutrients, especially if the pile of planting mix is large and heats up (like a compost pile).
BMP6: Fertilizer storage.Always store unused CRFs and other solid fertilizers in a dry, cool location.
BMP7: Electrical conductivity.EC is not well correlated with nutrient concentrations. Use EC only as a general guideline for troubleshooting, not for direct estimation of specific nutrient levels.
Donald Merhaut is Extension Specialist, Nursery and Floriculture Crops, Department of Botany and Plant Sciences, UC Riverside; Eugene Blythe is Associate Research Professor, Plant Soil Sciences - Coastal Research and Extension Center, Mississippi State University; Joseph Albano is Research Horticulturist, U. S. Horticultural Research Laboratory, Fort Pierce, FL; Julie Newman is Emeritus Floriculture and Nursery Crops Advisor, UC Cooperative Extension.
References
Merhaut DJ, Blythe EK, Newman JP, Albano JP. 2006. Nutrient release from controlled-release fertilizers in acid in a greenhouse environment: I. Leachate electrical conductivity, pH, and nitrogen, phosphorus, and potassium concentrations. HortScience41(3):780-787.
Blythe EK, Merhaut DJ, Newman JP, Albano JP. 2006. Nutrient release from controlled-release fertilizers in acid in a greenhouse environment: II. Leachate calcium, magnesium, iron, manganese, zinc, copper, and molybdenum concentrations. HortScience41(3):788-793.
Newman JP, Albano JP, Merhaut DJ, Blythe EK. 2006. Nutrient release from controlled-release fertilizers in a neutral-pH substrate in an outdoor environment: I. Leachate electrical conductivity, pH, and nitrogen, phosphorus, and potassium concentrations. HortScience41(7):1674-1682.
Albano JP, Merhaut DJ, Blythe EK, Newman JP. 2006. Nutrient release from controlled-release fertilizers in a neutral-pH substrate in an outdoor environment: II. Leachate calcium, magnesium, iron, manganese, zinc, copper, and molybdenum concentrations. HortScience 41(7):1683-1689.












