Steaming And Other Management Practices For Pre-Plant Weed Control In Nurseries
by Steven A. Fennimore
Introduction
Weed seed are the means by which annual weeds reproduce and disperse. The seed buried in the soil is referred to as the seedbank. Most seed in the soil seedbank were produced in the same field or greenhouse. Some of the seed in the seedbank moved there through the actions of wind, water, animals or the activities of man. Annual weeds usually regenerate from seed stored in the soil seedbank. The seedbank reflects the effectiveness of recent weed management practices in the field or greenhouse and will determine future weed infestations. This article will outline some of the factors that influence weed seedbanks and how to use steam to kill weed seeds.
Weed Seedbanks
Harper (1977) viewed the soil seedbank much as a bank account to which deposits and withdrawals can be made (fig. 1). Deposits occur as weed seed enter the seedbank from local production or dispersal. Withdrawals occur by germination, death and consumption by birds or insects. Only a small fraction of the seedbank is capable of germinating at any given time.
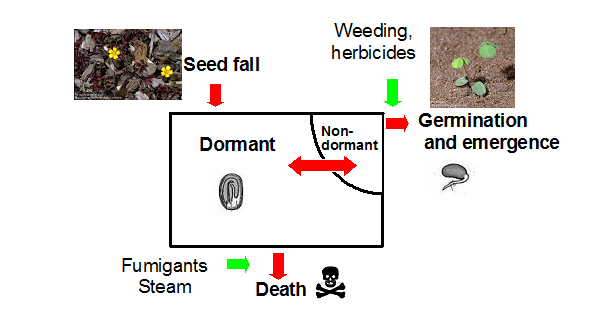
Fig. 1. Flow chart for the dynamics of weed seeds in the soil (Harper 1977).
When we discuss greenhouses we are not talking about “weed seedbanks” as they exist in an agricultural field, but weed seed that are anywhere in the greenhouse — under the bench, in the gravel under pots and in the soil or potting mix. The ecosystem in a greenhouse is much less variable than in an open field, but many of the concepts that weed ecologists have developed to talk about weed seedbanks in the field hold true for greenhouses. Generally seedbanks are composed of a few weed species that make up 70% to 90% of the total. A second group of species comprises 10% to 20% of the seedbank, but is not adapted to the current production system. The final group of seed consists of newly introduced species and seed from previous crops (Wilson 1988).
Soil seedbanks are what we target when we use soil fumigants or steam to disinfest soil. We use steam to eradicate the seedbank in the soil mix. However, after steaming or fumigation, the potting mix can become reinfested with weed seed. Many weed species are well suited for dispersal into greenhouses by wind from uncontrolled weeds surrounding the greenhouse, or by human-aided dispersal such as muddy work boots or tires. If we utilize cultural practices that minimize introduction of weed seed into the greenhouse by using preventative practices such as controlling weeds in and around the greenhouse, we practice preventative weed management rather than reactive weed control. A grower who does not tolerate weed seed set in and around the greenhouse minimizes the risks of higher production costs due to higher handweeding costs. For example a grower with a relatively weed-free greenhouse may have lower production costs due to lower hand-weeding bills.
Additions to the Seedbank
Seed can enter the seedbank by many means, though the largest sources are weeds producing seed within the field (Cavers 1983). Most seed in the seedbanks of farmland came from annual weeds growing on that same land (Hume and Archibold 1986). Just as in open agricultural fields, most weeds that infest greenhouses likely come from seed that were produced in the same greenhouse. Individual weeds can produce large numbers of seed when grown without competition (Table 1). I do not have the data for greenhouse weeds, but the concepts are the same — if weeds are given the chance to set seed they will.
Table 1. Seed production and seed survival (Wilson 1988).
| Weed species | No. of seed produced per plant (Stevens 1954, 1957) |
| Common lambsquarters | 72,450 |
| Common purslane | 52,300 |
| Common ragweed | 3,380 |
| Pennsylvania smartweed | 19,300 |
| Prickly lettuce | 27,900 |
| Redroot pigweed | 117,400 |
| Shepherd’s-purse | 38,500 |
Weed seed can enter a field from external sources such as mud on equipment or shoes, contaminated crop seed, animals, wind, and manure. Many weed seeds have special attachments that allow them to be dispersed by wind, water or animals (Fig. 2). Wind dispersal (Fig. 2 a–d) allows a few seed to move great distances, however, most seed remain close to the mother plant. Windblown seed such as common groundsel can easily blow into the greenhouse from surrounding fields. The introduction and dispersal of noxious weeds is the greatest threat from dispersed seed.
Seed Losses
Although seed of many weed species have the potential for long-term survival in the seedbank, most seed have a short life (Murdoch and Ellis 1992). Factors accounting for the loss of weed seed in the soil include germination, decay and predation. The relative importance of each factor varies with species and environmental conditions (Buhler et al. 1997). Fumigation and steam are also means of accelerating the loss of viable seeds in the seedbank (fig. 1).
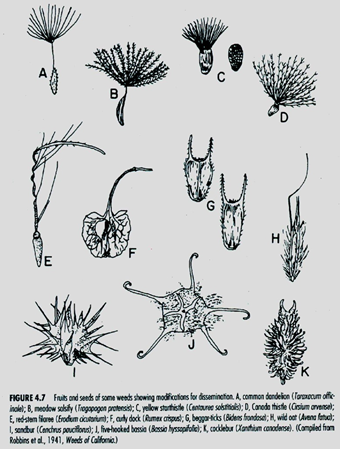
Fig. 2. Characteristics that aid dispersal of weed seed (Robbins et al. 1941).
In a weed management program we are primarily interested in those seed that germinate and seedlings that emerge. Germinated weed seed can result in new plants that may reduce crop yields and require control. Most weed seed in the soil seedbank are dormant with a small fraction of nondormant seed capable of germination at any one time. Several types of dormancy exist and most weeds possess one or more types (see insert).
From the moment a seed is shed its dormancy status is one of the key factors that determine when the seed will germinate. Seed dormancy is a means by which a plant species enhances its probability for successful reproduction in a changing environment. Dormancy is relieved by appropriate environmental conditions such as chilling, afterripening, light or scarification. Embryo dormancy is often reversible and represents a flexible system that allows a weed seed to adapt to its environment. The induction of secondary dormancy is the response of many weeds species to unfavorable environmental conditions. Secondary dormancy and weather conditions are responsible for much of the variation in weed germination from year to year.
Weed Management
Weed seed densities can be greatly reduced by eliminating seed production for a few years; conversely, soils with low seed densities can be quickly reinfested with weed seed if plants are allowed to produce seed. Burnside et al. (1986) found that broadleaf and grass seed density declined 95% after five weed-free years. In the sixth year, herbicide use was discontinued and seedbank density rebounded to within 90% of the original density. Although seed production from most weed species can be reduced by management factors, seed production will likely remain high enough to maintain or increase the seedbank with low to moderate weed infestations. Hartzler found that velvetleaf grown at densities of 2 and 4 plants per 100 square feet and allowed to set seed in year 0 resulted in as many as 1,800 plants per 100 square feet during years one to four, even though no velvetleaf plants were allowed to set seed during that period (Hartzler 1990).
Weeds can survive in a greenhouse either by using seed dormancy or seed dispersal to allow some individuals to escape control and produce seed. Seed dormancy is a characteristic that allows weeds to survive. In a weed species that has seed dormancy, weed seed germinate at a low rate over long periods of time, increasing the chance that a few individuals elude control and reproduce — thus replenishing the seedbank. Another weed survival strategy is dispersal. With the dispersal strategy, some viable seeds find a safe place to reproduce. Management of weeds with seed dormancy requires reducing the seedbank population to low levels, such as with fumigants or steam sterilization, and then maintaining strict weed control measures indefinitely to prevent reestablishment of the weed population. With weeds that have seed that disperse widely, the seed population in the greenhouse seedbank must be reduced and survivors controlled. At the same time the surrounding area must be kept as weed-free as possible to reduce the incidence of new weed seed dispersing into the greenhouse.
Preemergence herbicides kill germinating seeds and therefore act on only a small portion of the soil seedbank. Similarly, postemergence herbicides and tillage can only kill emerged weeds. Therefore, most of our weed control tools do not affect the dormant weed seeds in the soil seedbank. There are some exceptions: soil fumigants and steam can act on the entire seedbank including dormant and nondormant seed (fig. 1).
Steaming. Steam heating uses heat to kill weed seeds. In this process, conventionally used in greenhouse beds and in soilless media for container production, steam is mixed with air and injected into the media to heat it to 180°F for 30 minutes (Baker 1957). Length of time and temperature are critical if weed seeds are to be controlled. The pile or bed must be covered with a tarp so that the entire area, including the outer edges, reaches 180°F (Wilen and Elmore 2009; Baker 1957). The moisture of the media to be steam sterilized is also important — uniform heating is necessary if we are to kill weed seed throughout the media batch, and moist media conducts heat more readily than dry media. Further, weed seed are more easily killed when imbibed with moisture. This includes ungerminated weed seed that are swollen with high water content, which facilitates heat conduction from the seed surface to the embryo, and imbibed weed seed that germinate in the moist media.
There has been increased interest in the use of steam in field applications due to the phase out of methyl bromide. My research has shown that heating to 158°F for 20 minutes is effective in killing weed seeds in the field. As with steaming greenhouse beds and container media, proper moisture levels are important. Further, soil clods should be avoided as it is difficult for steam to penetrate the clods.
In trials conducted near Salinas and Watsonville in 2007 to 2009, we demonstrated that steaming in the field was comparable to fumigation treatments (methyl bromide/chloropicrin or chloropicrin/1,3-dichloropropene). However steaming with traditional pipe and hose methods of distribution is too expensive for commercial use. One strategy to reduce costs is to use an automatic applicator to increase steam application efficiency. Because there are no automatic steam applicators that are commercially available for raised beds, which are utilized by California strawberry and cut flower growers, we developed an “alpha” prototype (Clayton Steam Generator) in September 2011. This prototype was successful in heating the top 24 inches of the soil profile in beds for 20 minutes above 158°F. Although less expensive than traditional steaming methods, the operating cost of the alpha prototype is still too high ($5472 per acre). We plan to take what we have learned from the “alpha” prototype and design a more efficient commercial “beta” prototype in 2012. Our objective is to make field steaming as comparable in cost to methyl bromide fumigation as possible ($3500 per acre in California).
Crop Rotation. Crop rotation is effective for weed management because changing patterns of disturbance diversifies selection pressure. This diversification prevents the proliferation of weed species well suited to the practices associated with a single crop. To better manage weeds one needs to change practices regularly. For example, if you are growing a container plant that requires two years to prepare for the market, this is plenty of time for weeds to become adapted. It is convenient to leave the long-cycle crop in the same greenhouse, but a better strategy is to move a short- and long-cycle crop around so that the production cycle in a greenhouse is varied. Short crops provide frequent dry conditions in the greenhouse between crops that will kill weeds, and the empty greenhouse space between each crop cycle will allow the use of nonselective herbicides to kill weeds.
Conclusions
- Seedbanks are the source of most annual weed species.
- Most seedbanks are dominated by one or two species.
- Most weed seeds in the seedbank were produced in the same greenhouse.
- The greatest threat from weed seed dispersal is the introduction and spread of noxious weed species.
- Seed losses occur from germination, decay and predation.
- Dormancy is a key factor that determines when a seed will germinate and allows weeds to persist in the environment.
- A small number of weeds can produce many seeds and given the opportunity can restore the weed seedbank to high levels in a short time.
- Steam heating of soil or potting mix can kill dormant and nondormant weed seed.
- Steam for soil disinfestation in the field is as effective as fumigants — although it is more expensive than fumigants.
- Crop rotation minimizes the opportunities for one weed species to dominate a field or greenhouse.
Steven A. Fennimore is Cooperative Extension Weed Specialist, Department of Plant Sciences, UC Davis
References
Alrich RJ, Kremer RJ. 1997. Principles in Weed Management. Iowa State University Press, Ames, IA.
Baker KF. 1957. The U.C. System for Producing Healthy Container-Grown Plants. Manual 23. UC ANR Agric. Exp. Sta., Berkeley, CA.
Bewley JD, Black M. 1994. Seed: Physiology of Development and Germination (2nd ed). Plenum Press, New York, NY.
Buhler DD, Hartzler RG, Forcella F. 1997. Implications of weed seedbank dynamics to weed management. Weed Sci. 45:329-336.
Burnside OC, Moomaw RS, Roeth FW, Wicks GA, Wilson RG. 1986. Weed seed demise in soil in weed-free corn (Zea mays) production across Nebraska. Weed Sci. 34:248-251.
Cavers PB. 1983. Seed demography. Can. J. Botany. 61:3578-3590.
Wilen CA, Elmore CL. 2009. UC Pest Management Guidelines: Floriculture and Ornamental Nurseries – Container Nurseries. UC ANR Publication 3392. Available online at www.ipm.ucdavis.edu/PMG/r280701211.html.
Harper JL. 1977. Population Biology of Plants. Academic Press, London, UK.
Hartzler RG. 1996. Velvetleaf (Abutilon theophrasti) population dynamics following a single year’s seed rain. Weed Tech. 10:581-586.
Hume L, Archibold OW. 1986. The influence of a weedy habitat on the seedbank of an adjacent cultivated field. Can. J. Bot. 64:1879-1883.
Murdoch AJ, Ellis RH. 1992. Longevity, viability, and dormancy. In: Fenner M (ed.). Seeds: The Ecology of Regeneration in Plant Communities. CAB International, Wallingford, UK. p 193-229.
Robbins WW, Bellue MK, Ball WS. 1941. Weeds of California. California State Department of Agriculture, Sacramento,CA.
Wilson RG 1988. Biology of weed seed in the soil. In: Altieri MA, Liebman, M (eds). Weed Management in Agroecosystems: Ecological Approaches. CRC Press, Boca Raton, FL. p 25-39.