Field Evaluation Of Insecticides To Control Light Brown Apple Moth
by Steve Tjosvold and Neal Murray
Introduction and Goals
The light brown apple moth (LBAM) is an important quarantined pest in California, currently detected in 16 counties with a wide host range of native, ornamental and crop hosts (fig. 1). The quarantine has greatly affected the ornamental nursery stock industry along the central coast and other areas where LBAM is established in native and ornamental vegetation surrounding the nurseries where it is left unmanaged. This makes it difficult to keep LBAM from migrating into nurseries and re-infesting the production areas even with judicious control efforts. Regular official inspections occur at nurseries shipping nursery stock outside of the regulated areas. If LBAM is found in a nursery, insecticide treatments are usually required until LBAM is no longer found with follow-up inspections. (See link below for the CDFA official regulatory manual.) The larvae of this moth species, Epiphyas postvittana, are often difficult to distinguish morphologically from many other similar tortricid larvae that occur in ornamental crops, and therefore insecticides are often used proactively against any suspect larvae found by nursery staff or pest management scouts.

Fig. 1. LBAM female adult and egg mass. Photo S. Tjosvold.
Experiments at the South Australia Research and Development Institute (SARDI) screened insecticides, including horticultural oils for ovicidal activity against LBAM, using dip bioassays of exposed egg masses. Likewise, at the USDA Otis Lab (Buzzards Bay, MA), several insecticides targeting LBAM larval stages were screened in a laboratory environment. This information has been used by the California Department of Food and Agriculture (CDFA) to establish an official list of approved insecticide treatments for agricultural operations when required (see link below). Nursery operators usually choose to minimize the time lag between treatment and re-inspection so that the quarantined plants can be released for sale as quickly as possible. Usually the grower’s preferred treatment has been the officially designated “ovicidal” treatment containing one active product that targets larvae and a horticultural oil that targets eggs. So far there have been no studies to evaluate the efficacy of these insecticides and the prudence of combining the products in the field. Moreover, growers need to know how long these insecticides remain effective in the field so they can use good judgment as to how often spray treatments need to be applied. Since nurseries can often be re-infested by migrating moths, an important management strategy might be to target oviposition (egg laying), egg eclosion (egg hatch) and the development of the brood to the next generation.
The goal of this experiment was to determine the efficacy and residual action of many of the approved treatments and other new insecticides in preventing or retarding oviposition, egg development, egg hatch, and larva and pupa development in field conditions. Ultimately these experiments will aid in the development of an insecticide treatment strategy, with rotating chemical classes, to manage LBAM and prevent insecticide resistance in nurseries. This trial is being repeated in the summer of 2011 to strengthen our findings.
Materials and Methods
Plant Material. Pacific wax myrtle (Myrica californica) in 1-gallon pots were obtained from a commercial nursery in Watsonville, California. (In Santa Cruz County, Pacific wax myrtle is a common host of LBAM in the landscape.) Plants received no pesticides at least two months before the experiment began, and no supplemental fertilizer during the experiment. The plants were moved into quarantine field cages covered with 32 x 32 mesh Lumite Saran insect screen at a secure-access facility in Watsonville, California. Plants were pinched to create uniform plants and stimulate branching supportive of LBAM larval development, and held for 2 weeks before treatments. Temperature data was recorded inside one caged plant and degree day accumulation was calculated using a LBAM developmental model and temperature thresholds to help assess the development of the insects and time the treatments, moth infestations and evaluations in this experiment.
Insecticide Treatments. Treatments included many of those from the CDFA officially approved insecticide treatments, which represent a wide range of chemical classes with different modes of action. Several of the commonly used treatments by growers — spinosad, Bacillus thuringiensis (Bt) and methoxyfenozide — were applied alone, or combined with horticultural oil. All products were registered for ornamental nursery stock except emmamectin benzoate (registered for vegetables), chlorantraniliprole (registered for turf and landscape) and indoxacarb (registered for turf and landscape) (see Table 1). To insure thorough coverage of all treatments, a non-ionic surfactant (modified vegetable oil and organosilicone blend at 3 pints per 100 gallons) was added to all insecticides and the control, and all were applied at 200 gallons per acre (see Table 1).
|
Table 1. Insecticide Treatments and Rates |
||
|
Active ingredient |
Formulation |
Rate product / 100 gal. |
|
Bt (Bacillus thuringiensis) ssp. kurstaki |
54% DF |
16 oz. |
|
Bt ssp. kurstaki + horticultural oil |
|
16 oz. + 1 gal. |
|
spinosad |
11.6 % SC |
12 fl. oz. |
|
spinosad + horticultural oil |
|
12 fl. oz. + 1 gal. |
|
methoxyfenozide |
22.6 % F |
8 fl. oz. |
|
methoxyfenozide + horticultural oil |
|
8 fl. oz. + 1 gal. |
|
horticultural oil (petroleum oil) |
98% |
1 gal. |
|
lambda-cyhalothrin |
9.7 % GC |
5 fl. oz. |
|
emmamectin benzoate |
5% Powder |
4.8 oz. |
|
chlorantraniliprole |
18.4% SC |
4 fl. oz. |
|
indoxacarb |
30% WDG |
2.5 oz. |
|
diflubenzuron |
40.4 % SC |
4 fl. oz. |
|
Untreated Check |
|
|
Pre-oviposition bioassay. To assess the treatments’ residual efficacy, insecticide treatments were applied on August 24, 2010. Each treated plant was subsequently infested with 20 female and 20 male moths that were recently hatched, obtained from the LBAM colony at the USDA LBAM Project in Moss Landing, California. Plants were infested by enclosing each one in a 19 inch x 28 inch (48 centimeter x 71 centimeter) insect rearing bag (BugDorm by Megaview Science Co., Taiwan) made of white nylon netting (104 x 94 mesh) to securely contain all life stages on each plant. Plant blocks were infested at 4 successive days after treatment (DAT) with insecticides: 1 DAT, 7 DAT, 14 DAT, and 21 DAT (respectively: Aug. 25, 2010; Aug. 31, 2010; Sept.8, 2010, Sept.14, 2010).
Post-oviposition bioassay. To assess the treatments’ efficacy when applied to recently laid egg masses, the insecticide treatments were applied after 10 days (225 degree days accumulated, on October 1, 2010) when most egg masses were laid and adults had died. Plants were infested with fertile moths on Aug. 21, 2010 as described above and treated 10 days later.
Evaluation of egg laying and eclosion. Approximately 3 weeks after each moth infestation (455 to 543 degree days), when all eggs had apparently hatched, the total number of egg masses per plant in the pre-oviposition bioassay were counted for 5 minutes by 2 persons (each counting one half of the plant). Then up to 3 leaves were randomly selected and pulled from each plant and examined with the aid of a stereo microscope to evaluate the number of eggs per egg mass, proportion of eggs that had successfully eclosed, and whether the eggs were laid on the bottom or top of the leaf.
Evaluation of survival from egg mass to larvae, pupae, or adults. For both pre- and post-oviposition bioassays, the brood developed very slowly in the field (over winter) for approximately 217 days (2750 to 2850 degree days) until most individuals had reached the adult stage. At this time, the total number of healthy life stages (larvae, pupae and adults) were counted on each plant.
Results and Discussion
Total larvae life stage survival. A random sample of all blocks and treatments in both bioassays indicated that all larvae were in late stages of development: 2.4% were 4th instar and 97.6 % were 5th or 6th instars.
Pre-oviposition bioassay. The overall average of eggs deposited on plants was 20.8 egg masses per plant. However, the average deposited at the 14 DAT (11.8 egg masses per plant) was significantly lower than the other dates. This is probably due to the particularly hot weather that occurred during oviposition which probably affected egg laying.
Of the leaves that were randomly sampled to evaluate egg eclosion and egg deposition location, the overall average number of eggs per egg mass for all treatments was 18. 6 on the bottom (B) of the leaves and 9.6 on the top (T) of the leaves, a 1.9 : 1 ratio (B/T). In contrast, the average number of eggs per egg mass for the control plants were 16.3 on the bottom of the leaves and 20.0 on the top of the leaves, a 0.8 : 1 ratio (B/T). The literature indicates the propensity of moths to deposit eggs on the top of the leaves as illustrated here in the control plants, but with the insecticides applied in greater concentration on the top of leaves, the moths preferred to deposit eggs on the bottom of the leaves.
The number of eggs deposited per plant was significantly lower in only the lambda-cyhalothrin treatment (1.4 egg masses per plant). Of those eggs that were deposited in that treatment, the average proportion of egg eclosion was also lower (data not shown).
The total surviving life stages were statistically lower (greater efficacy) for the Bacillus thuringiensis treatment when compared to the control treatment for up to 1 week after the treatment, but not for 2 or 3 weeks, which indicates a fairly short residual activity. The spinosad treatment showed significant efficacy as compared to the control for up to 2 weeks, but not for 3 weeks. The methoxyfenozide treatment showed significant efficacy as compared to the control for at least 3 weeks. In general, when horticultural oil was added to these treatments, efficacy was not improved, and in some cases, although not significant, was less efficacious. Horticultural oil (alone) showed inconsistent results on survival as compared to the control. Its uncertain activity could not be explained by repellency since egg number and egg number per mass were no different than the control. The lambda-cyhalothrin and emmamectin benzoate treatments showed significant efficacy for at least 3 weeks. The chlorantraniliprole treatment showed significant efficacy for 2 weeks but not for 3 weeks. The indoxacarb treatment showed significant activity for 1 week, but not longer. The diflubenzuron treatment in general showed no significant efficacy on total surviving life stages as well as on the other egg parameters that were evaluated (see fig. 2).
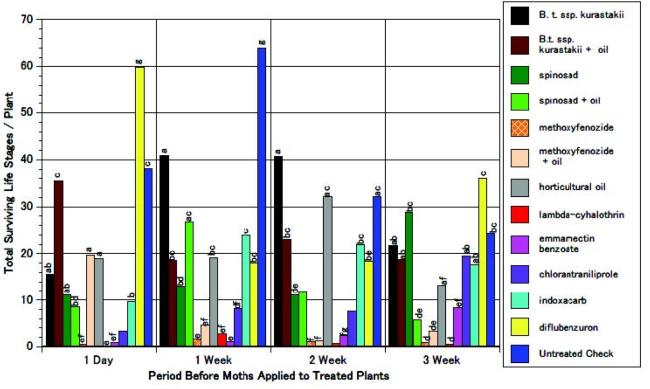
Fig. 2 Effect of insecticides applied before moth infestation (egg deposition) on the total surviving life stages
Post-oviposition bioassay. The total surviving life stages were statistically lower for all treatments except diflubenzuron. As with the pre-oviposition bioassay, there was no improvement of adding horticultural oil to the Bacillus thuringiensis, spinosad, or the methoxyfenozide treatment, although horticultural oil alone was somewhat effective (see fig. 3).
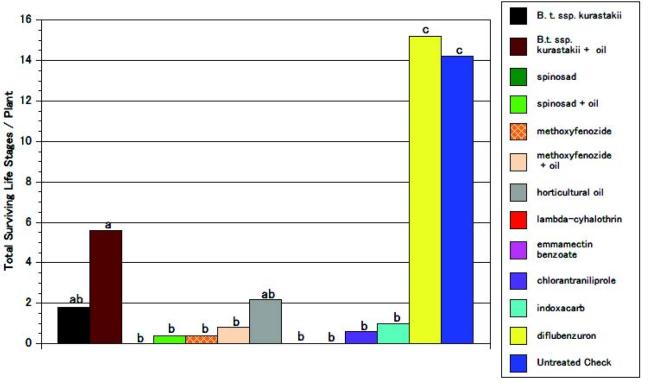
Fig. 3. Effect of insecticides applied after moth infestation (egg deposition) on total surviving life stages
Conclusions
The data shows that several insecticides can target egg-laying adults and subsequent development of the brood. These include several of the commonly used insecticides Bacillus thuringiensis and spinosad. The efficacy and longevity of methoxyfenozide was even better. Contrary to the recommendations of the officially approved list, adding horticultural oil did not improve control or longevity of Bacillus thuringiensis, spinosad, or methoxyfenozide. Lambda-cyhalothrin was highly effective and long lasting, affecting egg laying of adults by some mechanism, either by killing adults or inhibiting egg laying. This phenomenon will be investigated in the next experiment. There were also other effective and relatively long lasting treatments, including chlorantraniliprole and indoxacarb, newly registered turf and landscape insecticides, and emmamectin benzoate, which will be registered in the near future. Our data or specific experimental conditions cannot explain the apparent ineffectiveness of diflubenzuron.
For nursery stock producers, Bacillus thuringiensis and methoxyfenozide would provide the most selective control of the current registered insecticides in this study since they are active specifically on Lepidoptera larvae. This may be important in some production systems because field monitoring studies using sentinel LBAM eggs have revealed that at least two naturally occurring species of the Trichogramma wasp (T. platneri and T. fasciatum) in California have an active role in parasitizing LBAM eggs within infested coastal areas. In addition, T. platneri (fig. 4) is being reared on a large scale and is being tested for efficacy as augmentative releases in the landscape and commercial nurseries, and might be used in the future as part of an integrated management program.

Fig. 4. Augmentative releases of Trichogramma platneri (shown parasitizing a moth egg) in landscapes and commercial nurseries are being evaluated as part of an integrated approach for the control of light brown apple moth.
Photo by Jack Kelly Clark. Source: UC Statewide IPM Project.
References
LBAM CDFA approved Insecticide list: http://phpps.cdfa.ca.gov/PE/InteriorExclusion/PDF/LBAMApprovedTreatments.pdf).
LBAM Regulatory Procedure Manual: http://phpps.cdfa.ca.gov/lbam/LBAMtoc.pdf
Steve Tjosvold is Environmental Horticulture Farm Advisor and Neal Murray is Staff Research Associate, UC Cooperative Extension, Santa Cruz County. The authors thank Dr. Greg Simmons (USDA, Moss Landing, CA) and his staff for their valued input into this project and their assistance in providing LBAM moths. Also, thanks to Dr. Scott Myers (USDA, Otis Lab, Buzzards Bay, MA) for helpful suggestions on experimental protocol and the selection of treatments.












